Recruitment requires consent, a single DNA sample and a very short online CRF
We recruit across all eligible groups and our full eligibility criteria can be found here: Entry criteria
We are approved to take consent in person, by telephone or in the case of retrospective recruitment, consent documentation can also be sent by post.
Our study documents can be found here: Study Documents
For patients lacking capacity - plan a review date to assess for regained capacity.
Regained capacity consent can be followed up bt telephone or in writing and we have an ethically approved letter available for this.
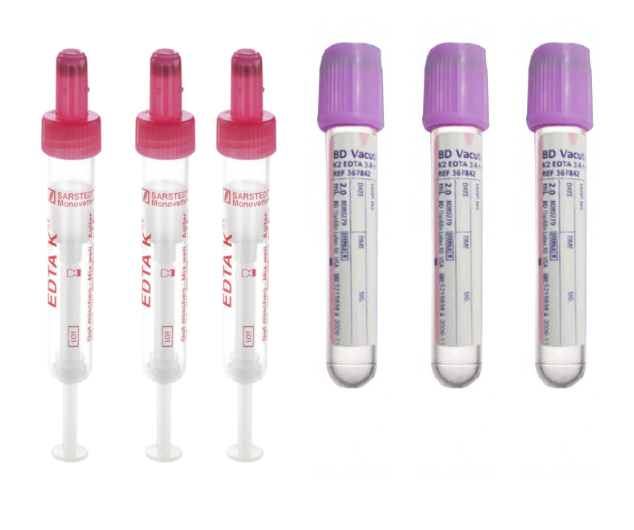
Blood is our preferred method of sampling and can be obtained in any EDTA blood tube. We send 9ml Vacutainer tubes as standard, but can supply monovette tubes if preferred and smaller volume tubes for paediatric units.
The amount of blood we need for each sample depends on the patient’s weight:
40kg: 9mls 20 to 40kg: 7mls 10 to 20kg: 4ml.
If a blood sample is not possible saliva may also be obtained. Please use only our approved oragene kits for this.
Take all four of the of GenOMICC sample stickers from inside one of the return GenOMICC specimen boxes that we have sent to you.
Label each blood or saliva tube with one of our GCC ID labels and put in the plastic bag with absorbent material, seal and package into the GenOMICC specimen box and return to the Wellcome Trust Clinical Reseach Facility (WTCRF), Edinburgh as soon as possible.
The specimen kits are already pre-labelled with a postage paid return address.
Samples will be stable for several days at ambient temperature so there is no problem posting samples on a Friday, over the weekend or during public holidays.
Use the patient ID stickers to label the consent form. The patient consent form, REDCap record and sample tube must all be created / labelled with the same GCC Number.
This means we can easily reconcile samples received at the lab, with the correct eCRF.
Destroy any unused stickers - the same number should never be used twice.
Store the consent form in the local site file, which should only be accessible by authorised staff, in a secure area.
RedCap CRF, including: - Clinical and demographic details - Positive microbiology (when available) - 60 day survival outcome (when available)
Any EDTA blood tube any size <=9ml